Y. Wang & J. W. Tang & W. Q. Ma &J. Feng & J. Feng
Abstract One hundred twenty crossbred piglets (Duroc × Landrace × Yorkshire) were used to determine the effects of dietary zinc glycine chelate on growth performance, tissue mineral concentrations, and serum enzyme activity. All pigs were allotted to four treatments and fed with basal diets supplemented with 0, 50, and 100 mg/kg Zn as zinc glycine chelate or 3,000 mg/kg Zn as zinc oxide (ZnO). After the 35-day feeding trial, results of the study showed that, compared to the control, average daily gain was improved (P<0.05) for pigs fed 100 mg/kg Zn from zinc glycine chelate or 3,000 mg/kg Zn from ZnO and Z from ZnO and Zn concentrations in serum and M. longissimus dorsi were significantly enhanced by 100 mg/kg dietary zinc glycine chelate and 3,000 mg/kg ZnO. In addition, supplementation of 100 mg/kg zinc glycine chelate decreased (P<0.05) the liver Fe level, liver Zn level, spleen Cu level, and kidney Cu level compared to that of the 3,000-mg/kg ZnO group. For feces mineral excretion, 3,000 mg/kg Zn from ZnO greatly increased the concentration of fecal Zn (P<0.01) and Mn (P<0.05) compared to that of the control or the 100-mg/kg zinc glycine chelate group. Moreover, alkaline phosphatase and Cu/Zn superoxide dismutase activities of pigs in 100 mg/kg zinc glycine chelate and ZnO treatments were greatly higher than that of the control. The results of present study showed that supplementation with zinc glycine chelate could improve growth and serum enzyme activities and could also decrease zinc excretion in feces in weanling pig compared to high dietary ZnO.
Keywords Zinc glycine chelate, Growth, Mineral concentration, Enzyme activity, Weanling pigs
Introduction
Zinc is an essential trace mineral and plays many significant roles in metabolism as a component of numerous metalloenzymes and transcription factors [1]. It is an activator or an integral part of the action of many enzymes and metalloenzymes, as well as being necessary for acid–base balance and the proper development of bone and cartilage [2, 3].
Several studies reported that pharmacological concentrations of inorganic zinc (1,000 to 5,000 mg/kg) added to the weanling pigs could improve growth and feed conversion [4–10]. However, extensive feeding of high levels of inorganic Zn leads to the excretion of excess Zn and impact on total mineral absorption and excretion, which could be potential deficits [11–14]. Fecal Zn excretion was markedly decreased when lower concentrations of Zn from organic sources were fed to weanling pigs as a replacement for pharmacological doses of Zn as zinc oxide (ZnO) [13, 14].
Research indicated that organic zinc had been proposed to provide a higher bioavailability than inorganic zinc in chicks [15, 16] and pigs [13, 17]. Case and Carlson [13] reported that pigs fed 500 mg/kg Zn as an organic Zn-polysaccharide (Zn-PS) complex had similar growth performance but excreted less Zn in the feces than pigs fed 3,000 mg/kg Zn as ZnO. Ward et al. [17] also reported that 250 mg/kg of Zn as Zn-methionine increased growth performance of weanling pigs equal to that of 2,000 mg/kg of Zn as ZnO.
Moreover, supplementation of weanling pig diets with 300 or 450 mg/kg of Zn as Zn-PS maintained phase 2 (d 14 to 35) and overall growth performance similar to that obtained with 2,000 mg/kg of Zn as ZnO [18]. Our previous study showed supplementation with appropriate zinc glycine chelate (Zn-Gly) can improve the growth performance and immunological capacity of broilers [16].
The objective of this experiment was to evaluate dietary ZnO and Zn-Gly on growth performance, tissue mineral concentrations, and serum enzyme activity in weanling piglets.
Materials and Methods
Animals and Experimental Design
One hundred twenty piglets (Duroc × Landrace × Yorkshire) with 7.68±0.81 kg body weight (mean ± SD) were allotted to four treatments, each treatment was replicated three times with ten pigs per replicate. Dietary treatments were designed with different supplementation of Zn as follows: (1) control (no Zn supplementation); (2) control + 50 mg/kg of zinc as Zn-Gly; (3) control + 100 mg/kg of zinc as Zn-Gly; (4) control + 3,000 mg/kg of zinc as ZnO.
Pigs were housed in concrete floored pens (ten pigs per pen) and fed a corn–soybean meal-based diet (Table 1) formulated to meet or exceed National Research Council nutrient requirement estimates [19]. In the 35-day study, each treatment was offered the respective diet and all pigs were given adhibited access to feed and water. Pigs (without feed for 12 h) and feed consumption were measured on days 0 and 35 in order to calculate the average daily gain (ADG), average daily feed intake (ADFI), and feed/gain ratio (F/G).
Blood, Tissue, and Feces Collection
At the end of feeding trail, 24 pigs (without feed for 12 h, six piglets of each treatment) were selected and humanely killed. Blood samples were drawn by jugular venipuncture into 10-mL tubes. Blood samples were centrifuged at 4 C for 15 min at 3,000 rpm and the serum thus obtained was used for enzyme activities and mineral analyses. The chops of M. longissimus dorsi taken from the loin eye area at the tenth and 11th rib were removed for mineral analysis. Liver, kidney, pancreas, and spleen samples were excised and immediately stored at−70 C until analysis for mineral concentrations. Fecal samples were collected, weighed, freeze-dried, ground in a stainless steel blender, and were individually frozen at−20 C until analysis for mineral concentrations.
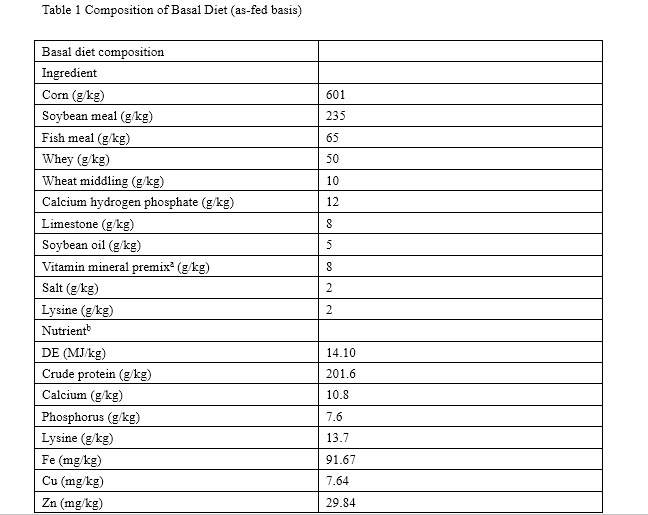
a Supplied the following per kilogram of diet: vitamin A, 15,000 IU; vitamin D 2, 3,000 IU; vitamin E, 30 IU; vitamin B 2, 5 IU; vitamin B 1, 3.0 mg; vitamin B12, 0.025 mg; biotin, 0.06 mg; pantothenic acid, 20 mg; nicotinic acid, 15 mg; Cu, 5 mg; Fe, 80 mg; Mn, 60 mg; Se, 0.67 mg; Co, 1 mg
b DE based on calculated values, others were analyzed values
Determination of Mineral Concentration
Fecal samples were prepared for mineral analysis using a method described by Armstrong et al. [20]. Briefly, fecal samples were digested using a microwave digestion system, dissolved with nitric acid, and then diluted with deionized–distilled water for analyses of minerals. Uniform samples were cut from tissues and minced with scissors. The pestle was mechanically driven at approximately 1,000 rpm and was moved up and down a minimum of five times until all tissue was broken up, then samples were wet-digested using nitric–perchloric acid and then diluted with deionized–distilled water for analyses of minerals [21]. Contents of Fe, Cu, Zn, and Mn were analyzed with flame atomic absorption spectrophotometry (AA-6300, Shimadzu, Tokyo, Japan).
Measurement of Cu/Zn-SOD and ALP Activities
Determination of Cu/Zn superoxide dismutase (Cu/Zn-SOD) activity was accomplished by our modification of the methods of Shaw et al. [22]. In brief, samples were mixed gently for 2 min and centrifuged (5,000×g, 15 min, 4 C). The clear supernatant was diluted appropriately with the reaction buffer (50 mM Tris–HCl, 1.0 mM diethylenetriamine Penta acetic acid, pH 8.2) and used to measure Cu/Zn-SOD activity. Units of Cu/Zn-SOD activity were expressed per gram of protein following Lowry protein determination of the hemolysate [23]. Assay for serum alkaline phosphatase (ALP) was measured as described by Tietz et al. [24]. In summary, 2.50 mL of alkaline buffered substrate solution was added to the reaction cuvette and warmed to 30 C. Then, 50 µL of the sample was added to the cuvette and mixed thoroughly to initiate the reaction. Immediately, the change in absorbance (∆A/∆t) of the reaction mixture at 405 nm at 30 C for up to 5 mm after initiation was recorded to calculate the ALP activity.
Statistical Analysis
Data were analyzed by analysis of variance as a randomized complete block design using the GLM procedures of SAS [25]. Pen was used as the experimental unit for growth performance data, and individual pigs were the experimental unit for other indices. The planned single degrees-of-freedom tests included the control vs. Zn-Gly (100 mg/kg Zn) and ZnO vs. Zn-Gly (100 mg/kg Zn) treatment. A significant level of 0.05 was used for estimating differences among treatments.
Results
Growth Performance
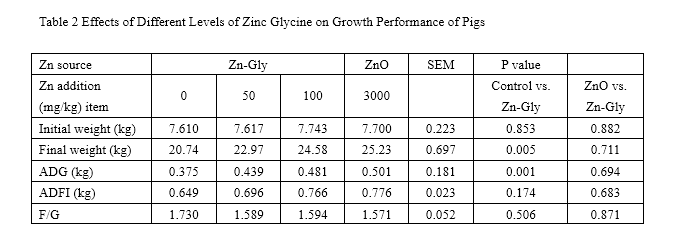
Nonorthogonal comparisons between the control vs. Zn-Gly(100mg/kg) and the ZnO vs. Zn-Gly(100mg/kg) treatments
SEM standard error of the mean, ADG average daily gain, ADFI average daily food intake, F/G feed/gain ratio
Table 2 displays the effects of ZnO and Zn-Gly supplementation on growth performance of weanling piglets. During the 0- to 35-day feeding period, both 100 mg/kg Zn-Gly and ZnO could improve the growth performance of swine in ADG (P<0.05). The ADFI of pigs was enhanced by addition with 3,000 mg/kg ZnO (P<0.05). No significant effect was observed on F/G among all the treatments (P>0.05). Moreover, there was no difference in improving growth performance between Zn-Gly and ZnO treatments (P>0.05).
Tissue and Fecal Mineral Concentration
Serum and Tissue Mineral Concentration
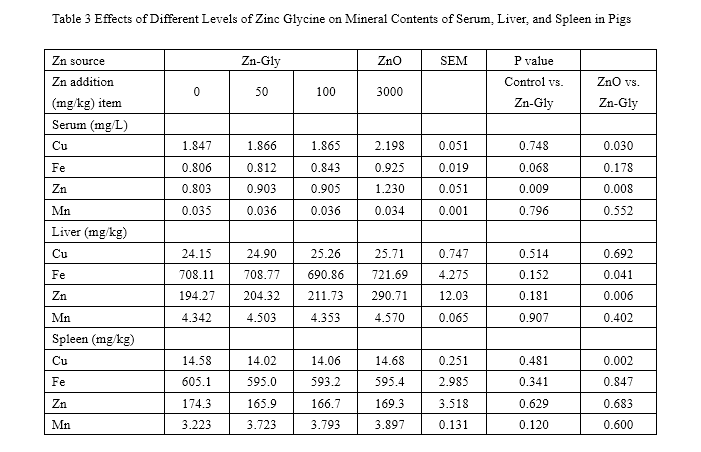
Nonorthogonal comparisons between the control vs. Zn-Gly(100mg/kg) and the ZnO vs. Zn-Gly(100mg/kg) treatments
SEM standard error of the mean
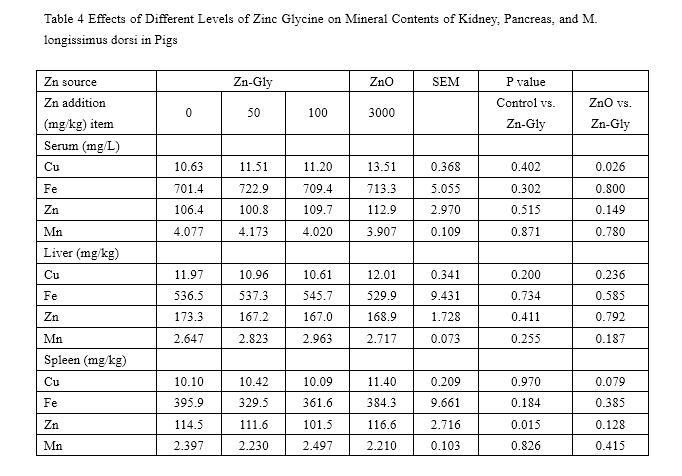
The effects of ZnO and Zn-Gly on serum and tissue mineral concentration are presented in Tables 3 and 4. During the 0- to 35-day feeding trail, compared with the control, the concentration of Zn in serum was significantly enhanced (P<0.05) by addition with 100 mg/kg Zn-Gly or 3,000 mg/kg ZnO. However, compared to those of the control, supplementation of Zn-Gly did not greatly affect Cu, Fe, and Mn concentrations in serum.
The pigs of the ZnO group have higher serum Zn and Cu concentrations (P<0.05) than that of the 100-mg/kg Zn-Gly group.
Nonorthogonal comparisons between the control vs. Zn-Gly (100 mg/kg) and the ZnO vs. Zn-Gly (100 mg/kg) treatments
SEM standard error of the mean
Compared with the control, adding additional 100 mg/kg Zn-Gly did not significantly enhance the levels of tissue mineral concentrations, expect for M. longissimus dorsi Zn concentrations. In addition, supplementation of 100 mg/kg Zn-Gly decreased the liver Fe level (P=0.041), liver Zn level (P=0.006), spleen Cu level (P=0.002), and kidney Cu level (P=0.026) compared to that of the negative control (3,000mg/kg ZnO group). Liver Zn and Fe storage, kidney Cu storage, and M. longissimus dorsi Cu storage was improved when pigs were fed 3,000 mg/kg Zn as ZnO compared to those of the control.
Feces Mineral Excretion
Nonorthogonal comparisons between the control vs. Zn-Gly (100 mg/kg) and the ZnO vs. Zn-Gly (100 mg/kg) treatments
SEM standard error of the mean
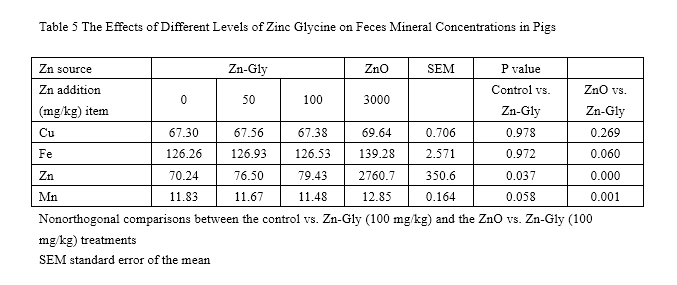
Concentration of Cu and Fe in feces (Table 5) had no significant difference among all treatments (P>0.05). Feces Zn level was enhanced by supplementation with 100 mg/kg Zn from Zn-Gly compared to that of the control. Besides, 3,000 mg/kg Zn from ZnO greatly increased the concentration of fecal Zn (P<0.05) and Mn (P<0.05) compared to those of the control or of the 100-mg/kg Zn-Gly group.
Serum Enzyme Activities
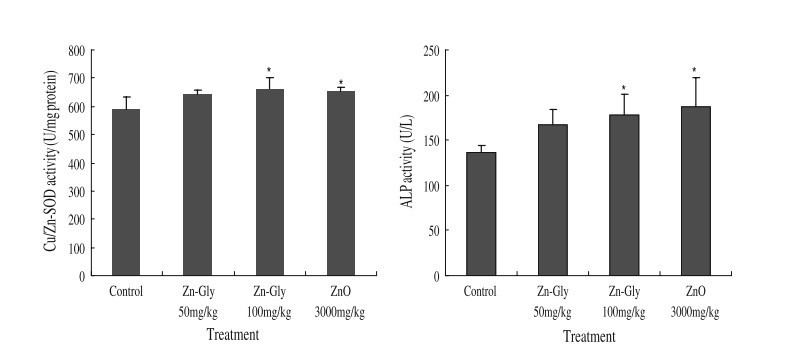
Fig. 1 The effects of ZnO and zinc glycine on serum enzyme activities in pigs. Control (no Zn supplementation), 50 mg/kg Zn-Gly group supplements with 50 mg Zn/kg diet from Zn-Gly, 100 mg/kg Zn-Gly group supplements with 100 mg Zn/kg diet from Zn-Gly, and ZnO group (positive control) supplements with 3,000 mg Zn/kg diet from ZnO. Asterisk indicates significant difference compared with the control
Figure 1 shows the ZnO and Zn-Gly on serum Cu/Zn-SOD and ALP activities in pigs. Supplementation of Zn-Gly (100 mg/kg) and ZnO improved (P<0.05) the activities of Cu/Zn-SOD and ALP compared to that of the control. However, no significant effects on the activities of Cu/Zn-SOD and ALP could be observed between Zn-Gly (100 mg/kg) treatment and ZnO treatment (P>0.05).
Discussion
Zinc is an essential dietary nutrient for swine. There is some evidence for improved bioavailability of organic compared to inorganic trace mineral sources. The present study showed that ADG was enhanced (P<0.05) for pigs fed 100 mg/kg of Zn from Zn-Gly. Ward et al. [17] reported that 250 mg/kg of Zn as Zn-methionine increased growth performance of weanling pigs equal to that of 2,000 mg/kg of Zn as ZnO. Case and Carlson [13] reported that pigs fed 500 mg/kg Zn as an organic Zn-PS complex had similar growth performance but excreted less Zn in the feces than pigs fed 3,000 mg/kg Zn as ZnO.
Feeding weanling pigs a diet containing 500 mg/kg of Zn as Zn proteins increased growth performance similar to that of pigs fed a diet containing 3,000 mg/kg of Zn as ZnO in two of three experiments [26]. Our previous study indicated that dietary Zn-Gly could increase growth in chicken [16]. It is well known that pharmacological levels of Zn (levels of approximately 300 to 3,000 mg/kg) are often used in the swine industry in the diets of nursery pigs immediately following weaning and have been reported to enhance growth performance [8, 13]. Our data support previous findings that excess dietary ZnO improved growth performance of the pigs. Current study also demonstrated that the 100-mg/kg Zn-Gly sources compared with the 3,000-mg/kg ZnO sources used in this experiment have no significant difference on growth performance in piglets. Hahn and Baker [2] reported that pigs fed 3,000 mg/kg Zn from ZnO had increased gain and feed intake. Cheng et al. [27] reported that supplementation of a weanling pig basal diet containing 32 mg/kg of Zn with 100 mg/kg of Zn from either ZnSO4 or Zn-Lys improved pig performance; however, no differences between Zn sources could be detected. Swinkels et. al. [28] supplemented Zn-depleted pigs with either ZnSO4 or a Zn amino acid chelate and observed that both sources of Zn had similar effects on pig performance.
The present study showed that the concentration of Zn in serum was significantly enhanced by addition with 100 mg/kg Zn-Gly or ZnO. Supplementation of Zn-Gly did not significantly enhance the levels of tissue mineral concentrations, expect for M. longissimus dorsi Zn concentrations compared to those of the control. The data were consistent with previous findings that plasma Zn concentration increased linearly with supplemental Zn [2, 29]. It is also reported that excessively high levels of Zn result in increased accumulation of Zn in soft tissue. Case and Carlson [13] reported increased levels of Zn in the liver and kidney of pigs supplemented with 3,000 mg/kg of Zn from ZnO but not in pigs supplemented with 500 mg/kg of Zn from ZnO. Schell and Kornegay [26] reported that pigs fed 2,000 mg of Zn/kg of diet as Zn-methionine had greater liver Zn and plasma Zn concentration than pigs fed 2,000 mg of Zn/kg of diet as ZnO. Higher bioavailability of organic zinc may have accounted for the high accumulation of Zn in plasma and soft tissue.
Although pig performance improves from feeding pharmacological concentrations of Zn as ZnO, there are environmental concerns associated with high concentrations of Zn in manure [30, 31]. Fecal excretion of Zn (in milligrams per day) is directly related to the quantity of Zn consumed (in milligrams per day) regardless of the Zn source [14]. As previously mentioned, Meyer et al. [31] reported an increase in fecal Zn excretion in pigs fed pharmacological ZnO (2,000 to 3,000 mg of Zn/kg of diet) for 21 days. Martinez et al. [32] reported that pigs fed 2,000 mg/kg of Zn as ZnO excreted 203 mg of Zn/day for the first14 days after weaning. Pigs fed Zn as Zn-Gly in our experiment similar to other experiments where pigs were fed organic sources of Zn [13, 14, 18] excreted markedly less Zn in the manure compared with pigs fed pharmacological concentrations of Zn as ZnO. Buff et al. [18] reported that zinc excretion was decreased to 76% by feeding 300 mg/kg of Zn as Zn-PS (406.4 mg/day) compared with 2,000 mg/kg of Zn as ZnO (1,714.3 mg/day).
Zinc is involved in many metabolic processes and has been found to be present in over 200 metalloenzymes including Cu/Zn-SOD and ALP [33]. Cu/Zn-SOD and ALP could be used as biological parameters to evaluate Zn status in the body [34]. The fact that ALP and Cu/Zn-SOD was affected by dietary zinc levels has been proved by many researchers [35, 36]. Present research also showed that ALP and Cu/Zn-SOD activities of pigs in the 100mg/kg Zn-Gly and ZnO treatments were greatly higher than that of the control. Zhang et al. [36] reported that dietary zinc levels significantly (P<0.01) affected serum Cu/Zn-SOD activity of chicken; its activity reached a peak with dietary zinc levels being 80–120 mg/kg diet. Revy et al. [37] found that ALP activity of pigs fed basal diet (containing 33 mg of Zn/kg) supplemented with 10, 25, 40, 60, or 80 mg/kg of zinc as sulfate increased linearly with zinc intake with a peak when dietary zinc reached 86 mg/kg. Current results means appropriate dietary Zn-Gly could affect the key enzymes associated with Zn and which mechanism needs further study.
Conclusion
The results of present study showed that supplementation with Zn-Gly could improve growth and serum enzyme activities and could also decrease zinc excretion in feces in weanling pig compared to pharmacological levels of ZnO. The results also indicate that Zn-Gly is a good source for zinc fortification in diets for weanling piglets.